Researchers have shown that the AREDS2 (Age-Related Eye Disease Study) dietary supplement formula, which has replaced antioxidants zeaxanthin and lutein for beta-carotene, reduces lung cancer risk as well as being more effective for reducing the risk of age-related macular degeneration progression, in comparison to the original formula.1✅ JOURNAL REFERENCE
DOI: 10.1001/jamaophthalmol.2022.1640
Because 2 studies found that lung cancer risk for smokers increased with beta-carotene, the objective with AREDS2 was to formulate a supplement that was equally effective and could be used by anyone, regardless of whether they smoked. This data gathered over 10 years not only confirms that the AREDS2 formula is safer, but also that it’s in fact more effective at slowing the progress of age-related macular degeneration.
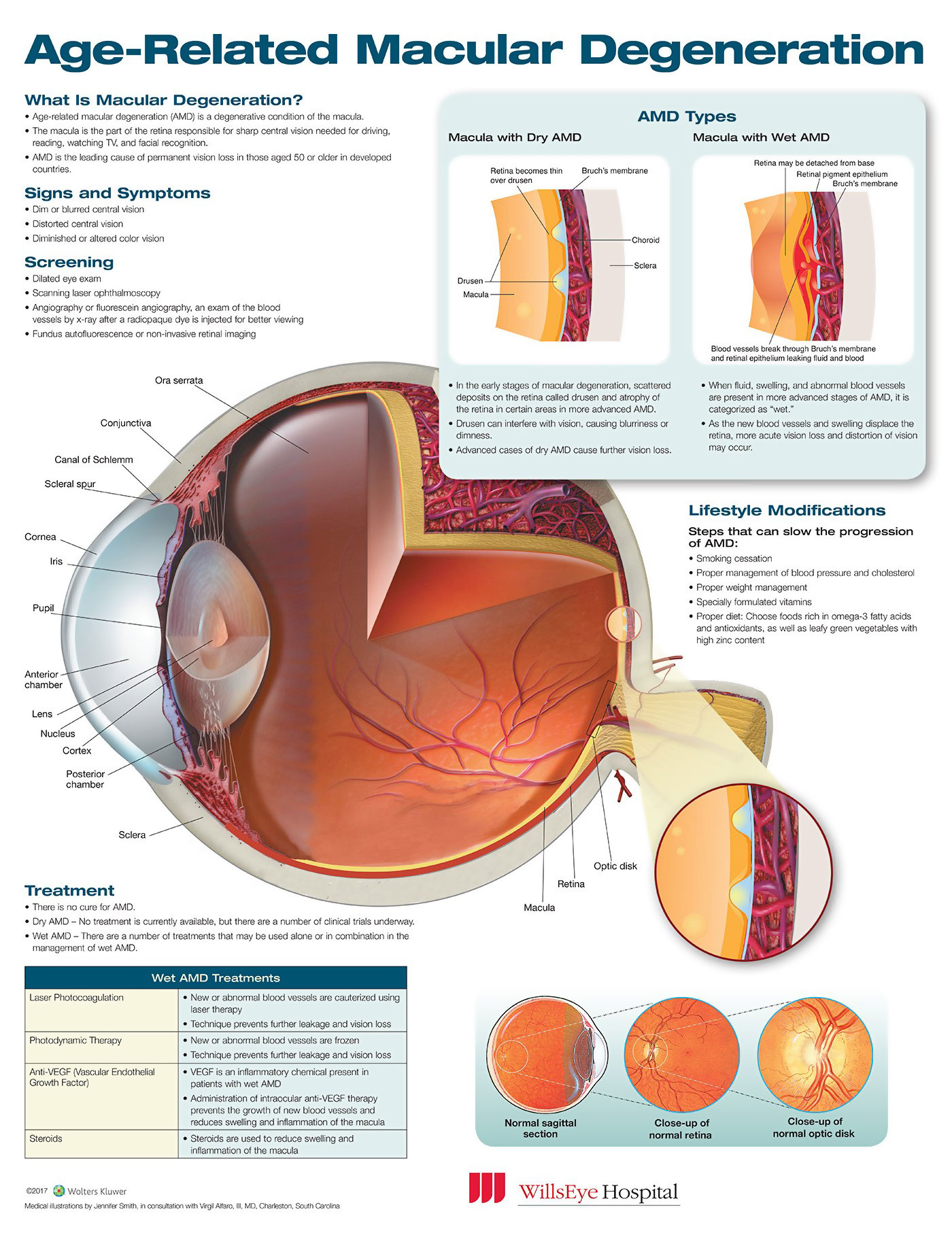
Age-related macular degeneration is a disease of the light-sensitive tissue known as the retina located at the back of the eye. Progressive retinal cell death in the macula, the section of the retina providing clear central vision, eventually results in blindness. Although treatment can delay or reverse vision loss, there is no existing cure for age-related macular degeneration.
The initial AREDS study demonstrated that a supplement containing 500 mg vitamin C, 15 mg beta-carotene, 400 international units vitamin E, 80 mg zinc, and 2 mg copper could slow age-related macular degeneration progression from moderate to late disease significantly. Two concurrent studies however also found that smokers taking beta-carotene had a considerably higher lung cancer risk than expected.
The AREDS2 study compared the formulation containing beta-carotene to one with 2 mg zeaxanthin and 10 mg lutein instead. Like beta-carotene, zeaxanthin and lutein are antioxidants with retinal activity. The formation containing beta-carotene was only given to individuals who had given up smoking or had never smoked.
At the conclusion of the 5-year AREDS2 study, it was determined that zeaxanthin and lutein didn’t increase lung cancer risk and that the new formation could reduce the progression risk of age-related macular degeneration by approximately 26%. After the study finished, the individuals were all offered the final AREDS2 formation that included zeaxanthin and lutein in place of beta-carotene.
3,883 of the original 4,203 individuals were followed up 5 years after the AREDS2 study ended to determine whether their age-related macular degeneration had progressed to late disease and if lung cancer had been diagnosed.
Although all the individuals had changed over to the zeaxanthin and lutein formula after the study ended, the follow-up study still found that beta-carotene increased lung cancer risk by almost double for individuals who had ever smoked. There was no elevated risk for lung cancer in individuals receiving zeaxanthin and lutein. Also, after 10 years, the group originally allocated to receive zeaxanthin and lutein had an extra 20% decreased progression risk to late age-related macular degeneration in comparison to individuals originally allocated to receive beta-carotene.